
NCERT Book for Class 11 Chemistry Part 1 – (Chapter 7 – Equilibrium)
Chapter 7 – Equilibrium
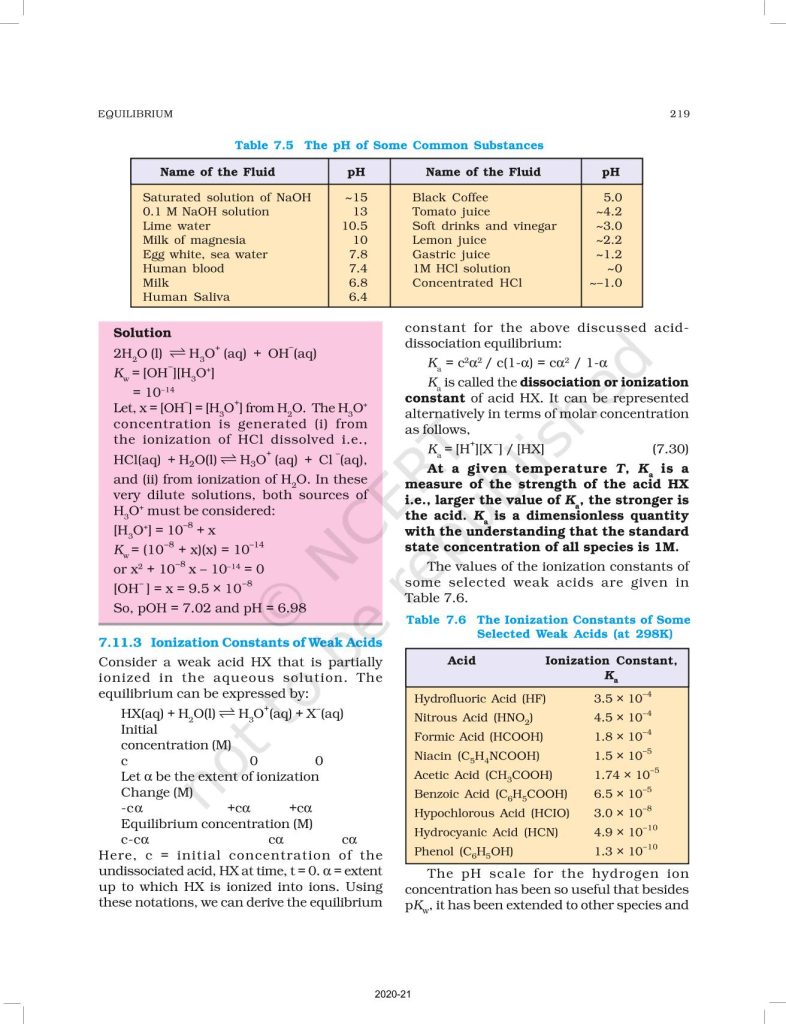
Chapter 7 – Equilibrium explains the state in which the rate of forward reaction equals the rate of backward reaction, resulting in no net change in concentration. It covers both physical equilibrium (like phase changes) and chemical equilibrium in reversible reactions. The chapter introduces the law of mass action and the concept of the equilibrium constant (Kc and Kp).
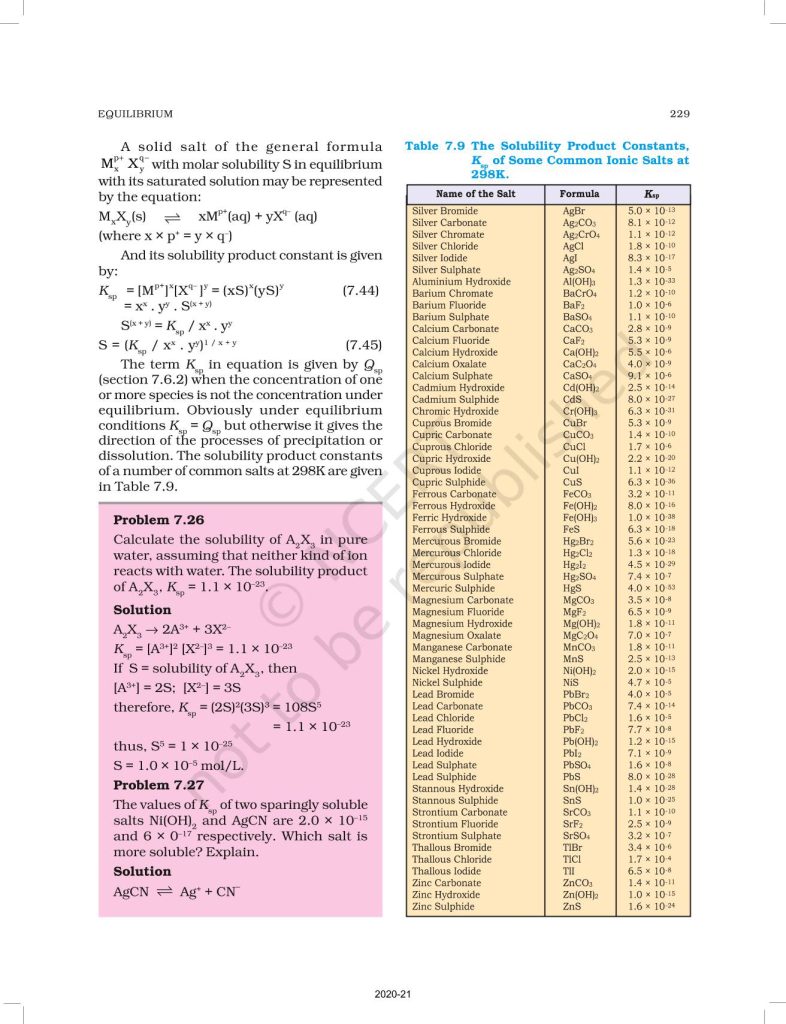
Students also learn about Le Chatelier’s Principle, which explains how equilibrium shifts due to changes in concentration, temperature, and pressure. The chapter further discusses ionic equilibrium, including acids, bases, pH, buffer solutions, and solubility product (Ksp). This topic is very important for CBSE board exams and competitive exams like NEET and JEE.
Equilibrium Class 11 NCERT









































- Part – 2







